- Home
- Research Highlights
Synthesis of New Cyclazines and 4,5-Diaryl-1H-pyrrol-3(2H)-one unit in Discoipyrroles from Indolizinone-DMAD Cycloadducts
Jais Kurian. and Muraleedharan K. M.*
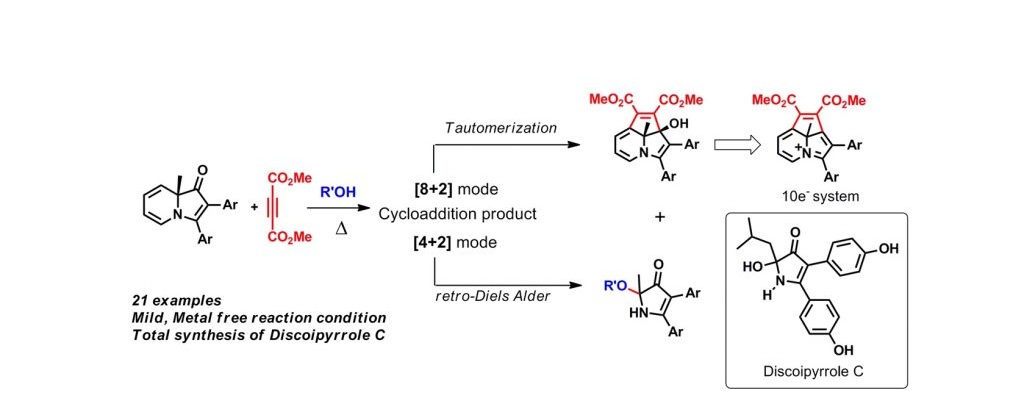
Reaction of indolizinones with Dimethyl acetylenedicarboxylate gave direct access to 3’,8a-dihydrocyclopenta[hi]indolizin-8a-ol and 1H-pyrrol-3(2H)-one in good yields. The former skeleton is a precursor to cyclazine with nitrogen on the periphery, a hitherto un-accessed 10-π system. Their formation involves initial [4+2] or [8+2] modes of cycloadditions; retro-Diels Alder reaction of [4+2] cycloadduct leads to 1H-pyrrol-3(2H)-one whereas [8+2] addition followed by π-reorganization lead to the azatricyle. Analysis of substituent effects on product distribution showed that electron donating groups on the C3-aryl ring promote the formation of azatricycle preponderantly. Treatment of one of these azatricycles (3c) with HBF4 led to the formation of the corresponding 10e-aromatic species which was detected by NMR spectroscopy. In addition, formation of 1H-pyrrol-3(2H)-one skeleton through normal retro-Diels Alder pathway was employed in the total synthesis of Discoipyrrole C, which is a new lead against lung cancer.
Org. Biomol. Chem. 2019, 10.1039/C9OB01655D
Electronically-tuned Triarylmethine Scaffold for Fast and Continuous Monitoring of H2S Levels in Biological Samples
Ramshad Kalluruttimmal, Divya Thekke Thattariyil, Archana Panthalattu Parambil, Ashis Kumar Sen, Lakshmi Chakkumkumarath * and Kannoth Manheri Muraleedharan *
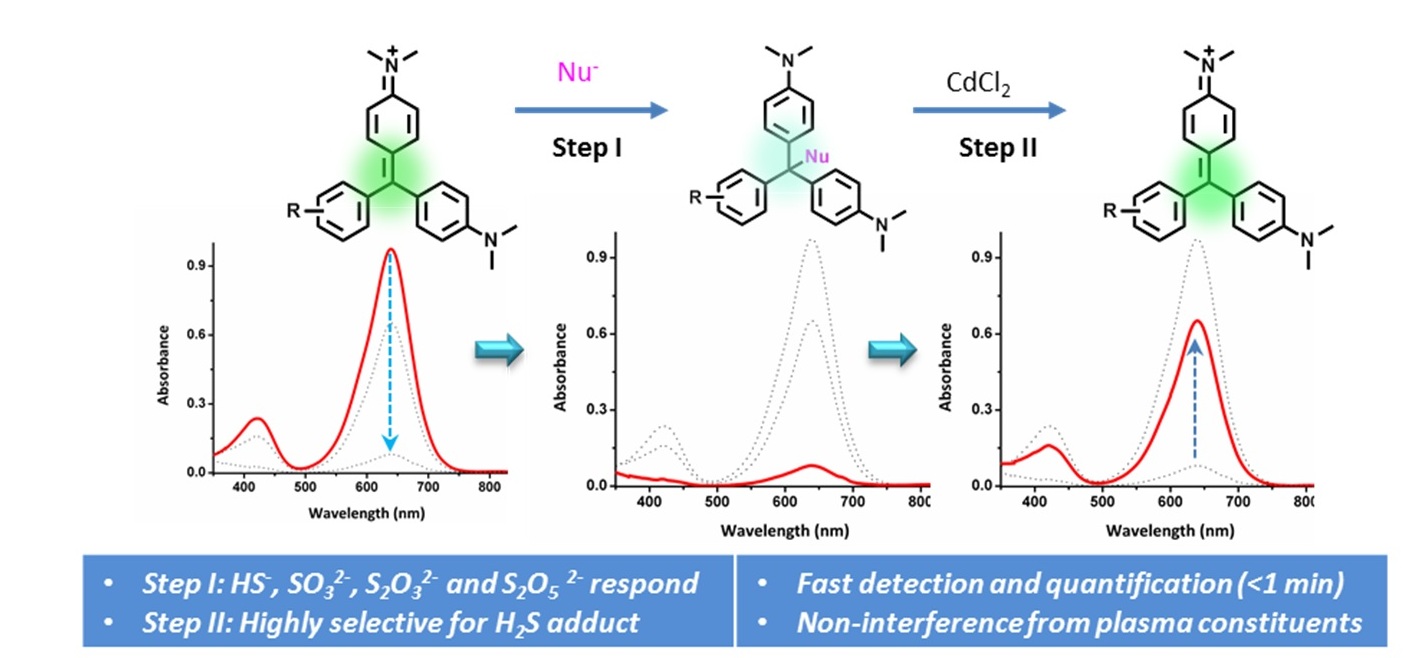
A sensor for the detection and quantification of H2S in biological samples should ideally meet a set of criteria such as: fast detection, high sensitivity in the desired concentration range, high selectivity, non-interference from biomolecules like proteins, ease of synthesis, long-term stability and water solubility. As part of a program to develop reliable chemical probes for continuous monitoring of this gasotransmitter in the blood plasma of SEPSIS-prone individuals in post-operative wards, we have looked at the possibility of improving the reactivity and selectivity profile of triarylmethine dyes towards different nucleophiles. After achieving high sensitivity through electronic control, the interference from sulfite, thiosulfate and metabisulfite was addressed by introducing a metal salt-mediated desulfuration step that results in dye regeneration selectively from its H2S adduct. The methodology optimized here meets the requirements mentioned above for real-life application.
Analyst, 2019, 144, 4210-4218
1-Hydroxymethyl-7-Oxabicyclo[2.2.1]hept-2-ene Skeleton in Enantiopure form through Enzymatic Kinetic Resolution
U. Chandrasekhar Reddy, and Kannoth Manheri Muraleedharan *
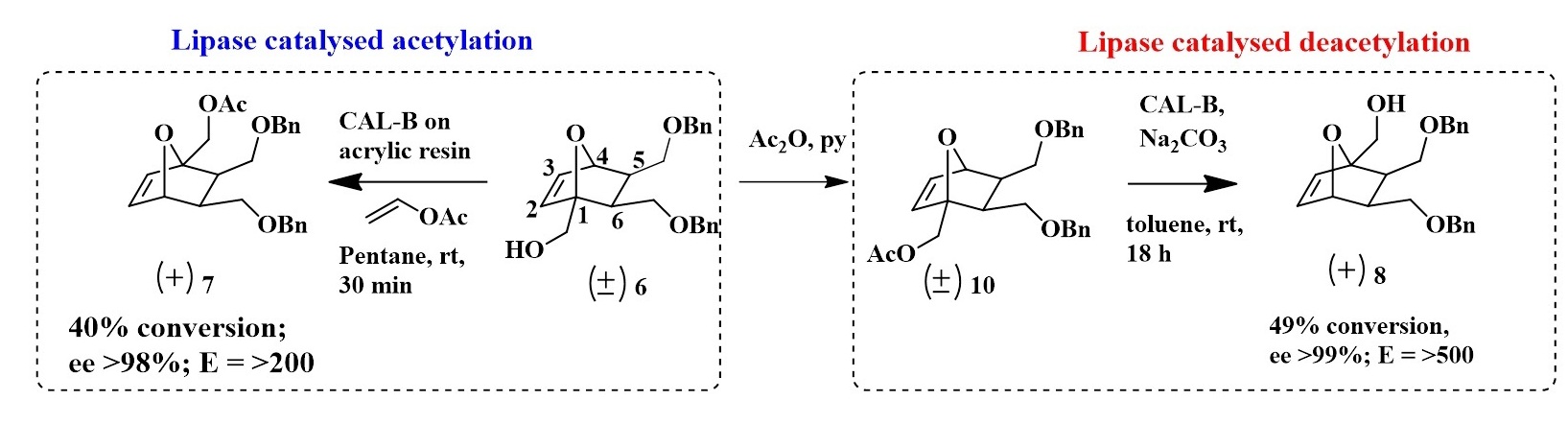
Enantioselective transesterification of 1-Hydroxymethyl-oxanorbornene derivative 6 was attempted with different lipases using vinyl acetate as the acyl donor. After systematic screening of lipases and solvents, CAL-B on acrylic resin in pentane was identified as the best system that gave >98% ee towards the (+) acetate with 40% conversion, in 30 min. Absolute configuration of this product was then confirmed by X-ray diffraction analysis of its 3,5-dinitrobenzoate derivative 9. To improve the yields, the reverse reaction, ie. deacetylation of the racemic acetate 10 was also studied. By systematic screening of lipases and optimization of reaction conditions, we identified CAL-B in toluene as most suited for this transformation, with 49% conversion and >99% ee in 18h.
Chirality, 2019, 31, 4, 336-347
Serine- and Threonine-derived Diamine Equivalents for Site-specific Incorporation of Platinum Centers in Peptides, and Anticancer Potential of these Conjugates
Sateeshkumar K., Kasipandi V., Soumya Saroj, Nalini V.,
Karunagaran D and Muraleedharan. K. Manheri *
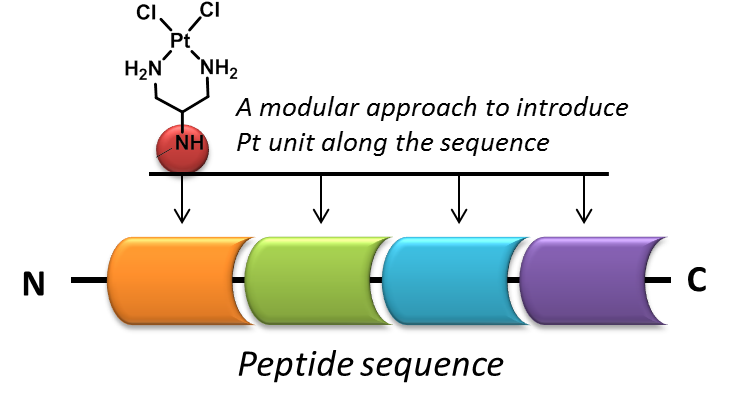
A modular strategy that allows introduction of one or more reactive platinum units at chosen locations along a peptide sequence is presented. This makes use of diazides generated from serine and threonine as diamine equivalents which can be conjugated to the peptide under standard coupling condition. Reduction of these diazides using Pd/C, H2 followed by platination affords the final products in good yields. Following this we have prepared a new class of peptide based cisplatin analogs and have carried out preliminary cytotoxicity evaluation and DNA interaction studies. Inclusion of lysine residues in the sequence was found to improve DNA interaction as well as anticancer activities compared to analogous conjugates with hydrophobic side chains
New J. Chem., 2018, 42, 2450-2458
Broad spectrum Anti-infective Properties of Benzisothiazolones and the Parallels in their Anti-bacterial and Anti-fungal effects
P. Gopinath, R. K. Yadav, P. K. Shukla, K. Srivastava, S. K. Puri, and K. M. Muraleedharan *
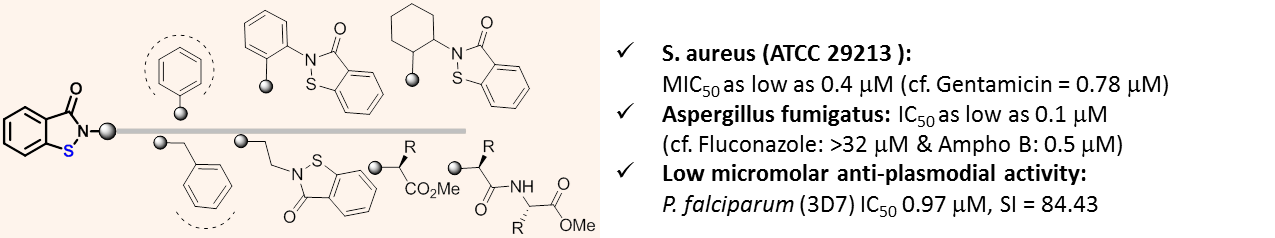
Various mono- and bis-benzisothiazolone derivatives were synthesized and screened against different strains of bacteria and fungi in order to understand the effect of multiple electrophilic sulfur atoms and substitution pattern in the immediate vicinity of reactive sulfur. Staphyllococcus aureus-ATCC 7000699, MRSA and S. aureus-ATCC 29213 (Quality Control strain) were more susceptible to this class of compounds, and the most potent derivative 1.15 had MIC50 of 0.4 mM (cf. Gentamicin = 0.78 mM). CLogP value, optimally in the range of 2.5-3.5, appeared to contribute more to the activity than the steric and electronic effects of groups attached at nitrogen. By and large, their anti-fungal activities also folowed a similar trend with respect to the structure and CLogP values. The best potency of IC50 = 0.1 mM was shown by N-benzyl derivative (1.7) against Aspergillus fumigatus; it was also potent against Candida albicans, Cryptococcus neoformans, Sporothrix schenckii, and Candida parapsilosis with IC50 values ranging from 0.4-1.3 mM. Preliminary studies also showed that this class of compounds have the ability to target malaria parasite with IC50 values in low micromolar range, and improvement of selectivity is possible through structure optimization.
Bioorg. MedChem. Lett. 2017, 27 (5), 1291-1295
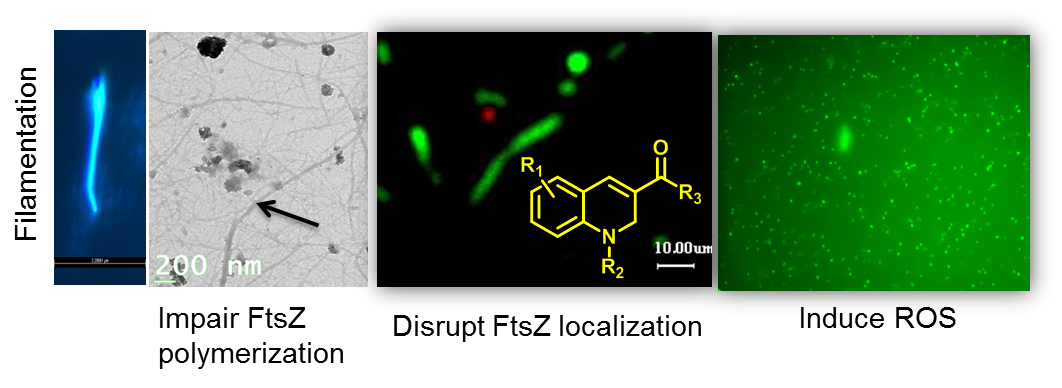
FtsZ inhibition and Redox modulation with one chemical scaffold: Potential use of Dihydroquinolines against Mycobacteria
Sridevi Duggirala, John Victor Napoleon, Rakesh P. N,Senu Adeeba V., Muraleedharan K. M.,* Mukesh Doble *
Napoleon John Victor , Janardhanan Gana and Kannoth Manheri Muraleedharan *
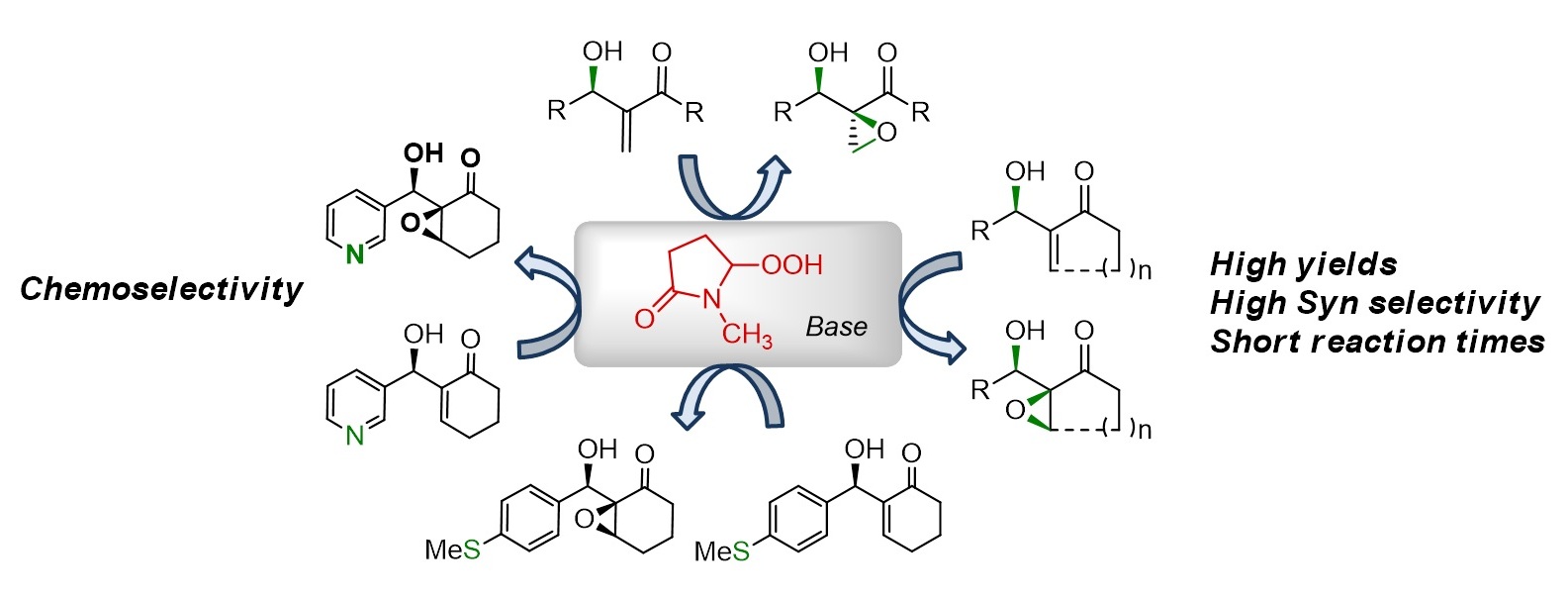
This report introduces NMPOOH/base as an excellent reagent system for hydroxy-directed syn selective epoxidation of electron-deficient olefins. High diastereoselectivity, short reaction times and remarkable chemoselectivity, especially in presence of oxidatively labile nitrogen or sulfur atoms make NMPOO- unique¯
Chem. Eur. J. 2015, 42, 14742-14747
An Expeditious and Metal-Free Synthetic Route towards Quinolones, Naphthyridones and Benzonaphthyridones
Napoleon John Victor and Kannoth Manheri Muraleedharan*
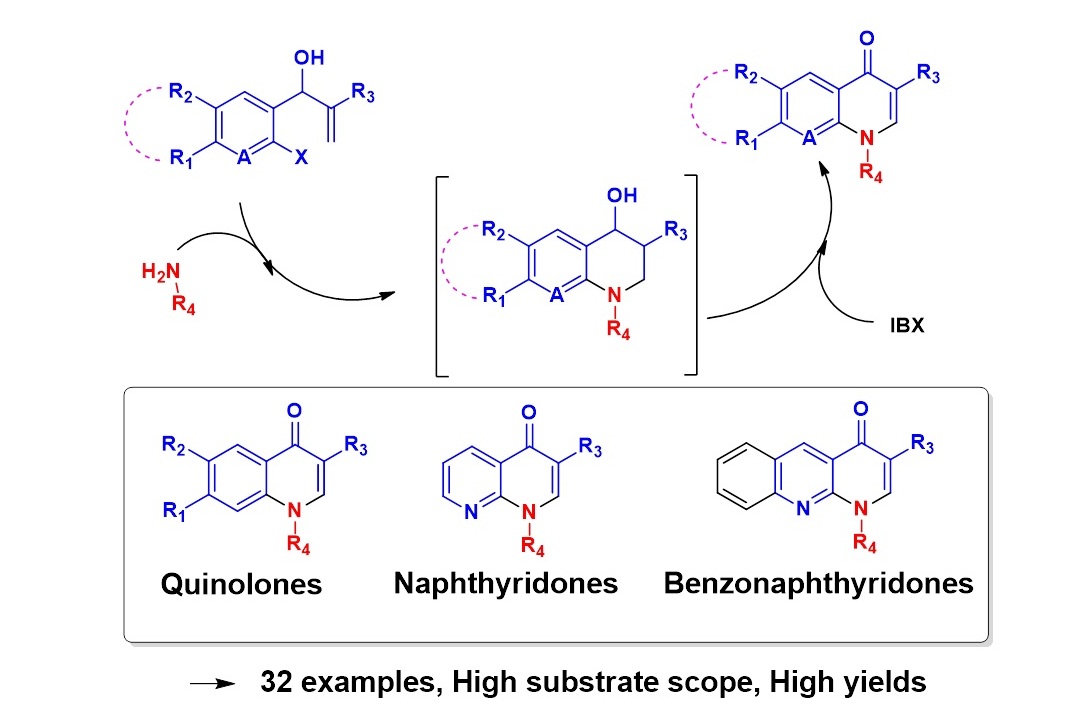
An efficient, two-step synthetic strategy has been developed to access quinolone-, naphthyridone- and benzonaphthyridone classes of chemotherapeutic agents from Baylis-Hillman adducts. The method involves tandem aza-Michael addition, SNAr cyclization followed by oxidation of the resulting 4-hydroxy-1,2,3,4- tetrahydroquinoline or 4-hydroxy-1,2,3,4-tetrahydro-1,8-naphthyridine derivative using IBX, and works well with substrates having a wide variety of substitution pattern.
Adv. Synth. Catal., 2014, 356, 17, 3600–3614
N-Substituted 1,2-Dihydroquinolines as Anticancer Agents: Electronic Control of Redox Stability, Assessment of Anti-proliferative Effects, and Mechanistic Insights
Napoleon John Victor, Ramasamy Sakthivel, Kannoth Manheri Muraleedharan,* and Devarajan Karunagaran*
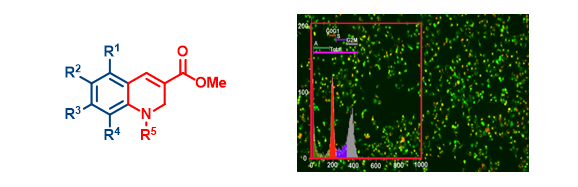
Anti-proliferative activities of a series of N-substituted 1,2-dihydroquinolines capable of causing redox imbalance in cancer cells are presented. Detailed studies showed that they arrest the cell cycle at the G2/M phase and induce apoptosis through an intrinsic pathway characterized by loss of mitochondrial membrane potential, DNA fragmentation, cytochrome c release, and activation of caspase-9 & caspase-
ChemMedChem, 2013, 8, 10, 1623–1628
Benzisothiazolones arrest the cell cycle at the G2/M phase and induce apoptosis in HeLa cells
Pushparathinam Gopinath, Krishnan Ramalingam, Kannoth Manheri Muraleedharan* and Devarajan Karunagaran*
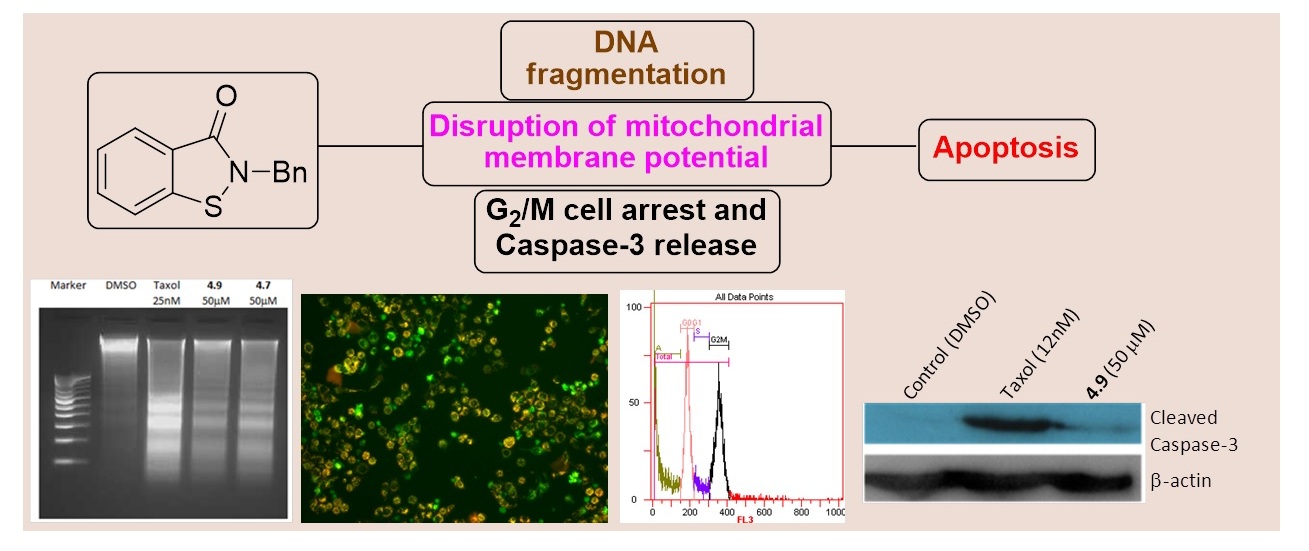
Anticancer activities of a series of benzisothiazolones having alkyl, aryl and aralkyl substituents on the nitrogen atom, and mechanistic basis of cytotoxicity are presented. Cellular responses like DNA laddering, disruption of mitochondrial membrane potential and caspase-3 activation on incubation of HeLa cells with representative compounds from this group suggested induction of apoptosis through intrinsic pathway. Their ability to arrest the cell cycle at the G2/M phase was confirmed by flow-cytometric analysis.
Med. Chem. Commun., 2013, 4, 749-752
Highly Chemoselective Esterification Reactions and Boc/THP/TBDMS discriminating deprotections under Samarium (III) Catalysis
Pushparathinam Gopinath, Surapaneni Nilaya and Kannoth Manheri Muraleedharan *
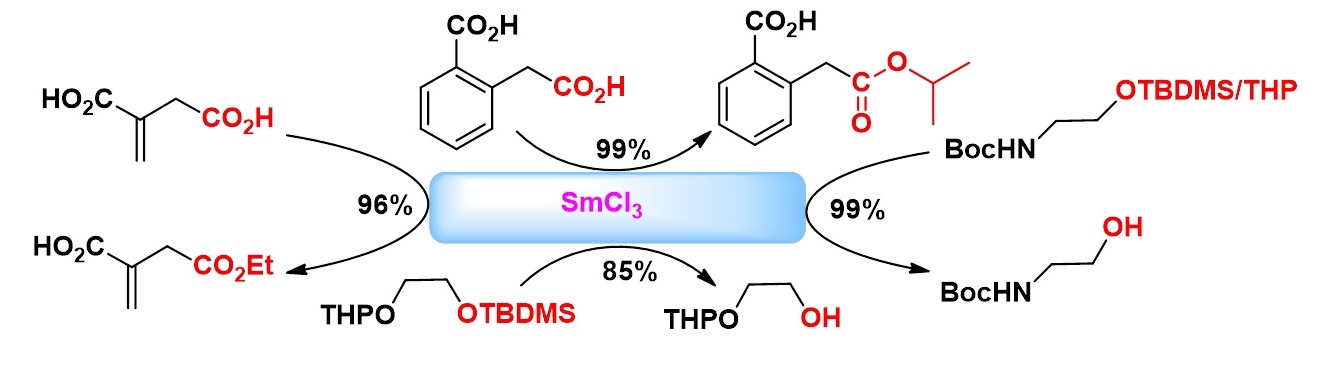
The usefulness of SmCl3 as an excellent catalyst for chemoselective esterifications, and selective removal of acid sensitive protecting groups such as Boc, THP and TBDMS in presence of one another, is demonstrated through suitable examples.
Org. Lett. 2011, 13 (8), 1932-1935
Direct Transformation of Baylis–Hillman Acetates into N-Substituted Quinolones Through an SN2’ → SNAr → (D3,4–D2,3 Shift) → Oxidation Sequence
John victor Napoleon and Muraleedharan Kannoth Manheri *
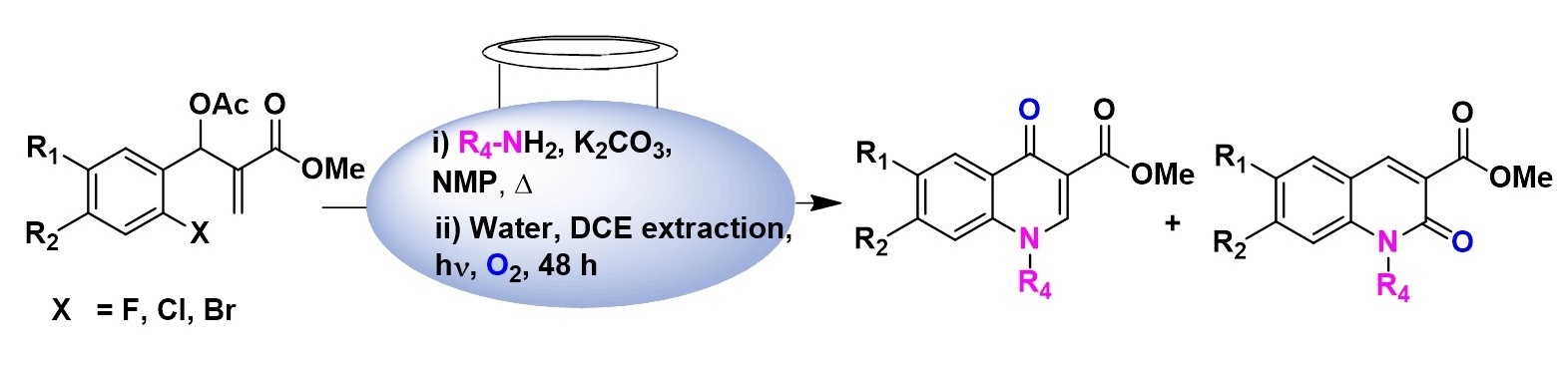
When subjected to tandem SN2’–SNAr cyclization in the presence of alkyl or aralkyl amines, Baylis–Hillman acetates gave the corresponding 1,2-dihydroquinolines, which on simple exposure to light and oxygen afforded the corresponding 4- and 2-quinolones through sensitized oxidation or a D3,4–D2,3 shift → oxidation cascade. The mechanism of the oxidation step, the stabilities of the 1,2- and 1,4-dihydroquinolines in solution and in the solid state, and the synthetic elaboration of the key intermediates to known therapeutic agents are discussed.
Synthesis, 2011, 20, 3379-3388
Synthesis of β- Hydroxyphosphonate and 1,2-Dihydroxy Acyclic Nucleoside Analogs via 1,3-Dipolar Cycloaddition Strategy
M. Ganesan and Kannoth M. Muraleedharan*
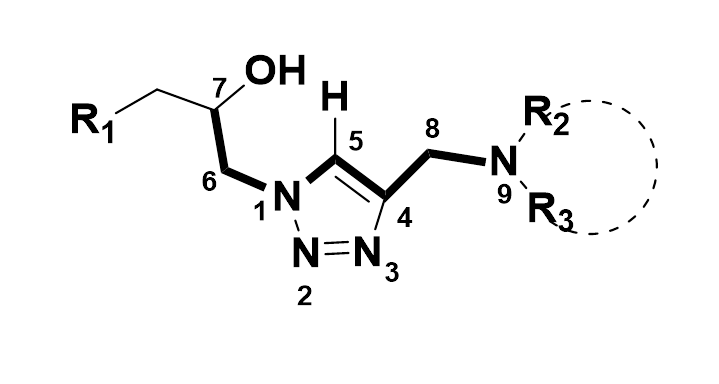
A convenient synthetic approach towards nucleoside analogs where b-hydroxyphosphonate- or 1,2-dihydroxy units are connected to the nucleic acid base through a triazole spacer is discussed.
Nucleosides, Nucleotides and Nucleic acids, 2010, 29 (2), 91-96
As many as six tandem reactions in one step! – Unprecedented formation of
highly functionalized benzothiophenes
Gopinath P., Nilaya S., Debiranjan Tripathy, Ramkumar V. and Muraleedharan K. M.*
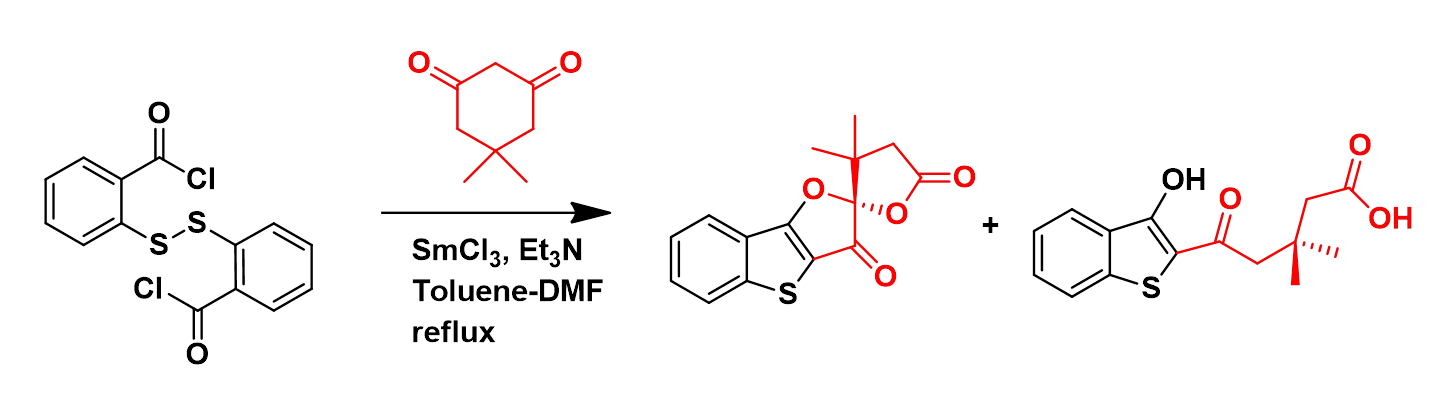
A novel reaction pathway involving 1,3-diketones and 2,2’-dithiodibenzoylchloride that gives access to benzothiophenes with spiroketal, lactone, carbonyl, hydroxyl and carboxylic acid functionalities is discussed.
Chem. Commun., 2009, 7131-7133
An efficient synthetic approach towards trans-b 2,3-amino acids and demonstration of their utility in the design of therapeutically important b 2,3-peptides and a,b 2,3-peptide aldehydes
Dhayalan Balamurugan and Kannoth M. Muraleedharan*
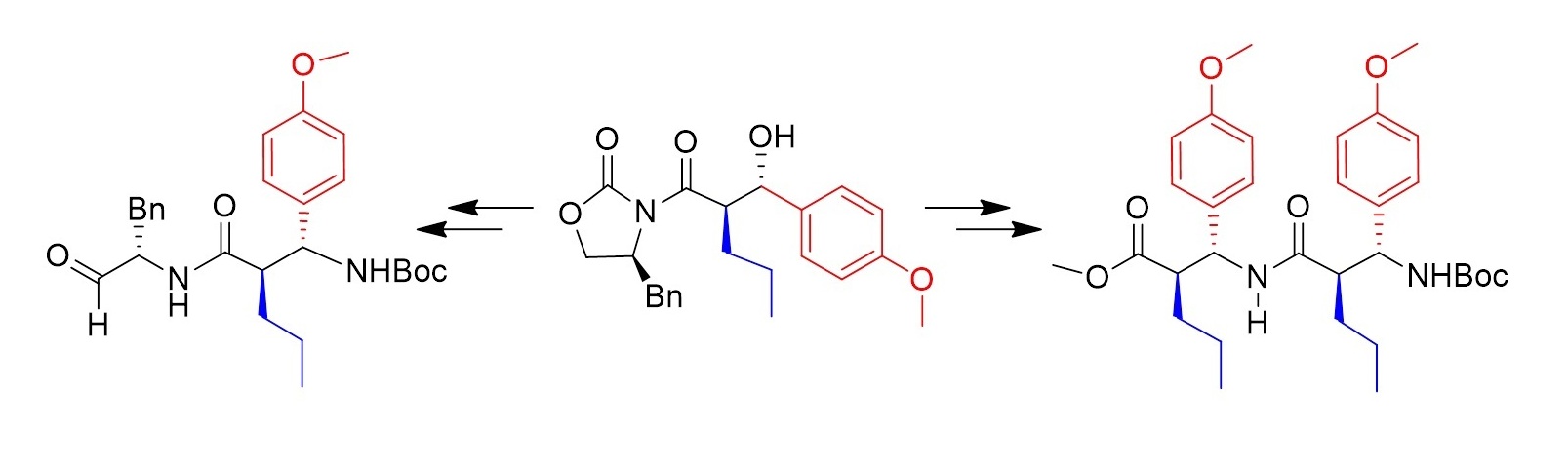
An efficient synthetic approach towards trans-b 2,3-amino acids involving anti-selective aldol, azidation and controlled hydrolysis as key steps is discussed. Apart from structural elaboration of these building blocks to homo- and hetero- dipeptides, possibility of selective endocyclic cleavage of the chiral auxiliary was advantageously used in the preparation of a,b-hybrid peptide alcohols and corresponding aldehydes which are promising candidates for biological evaluation against proteases of therapeutic interest.
Tetrahedron, 2009, 65, 10074-10082
Regio-selective lipase catalyzed hydrolysis of oxanorbornane-based sugar-like amphiphiles at air-water interface: A polarized FT-IRRAS study
Sarangi, Nirod Kumar; Ganesan, M.; Muraleedharan, K. M.; Patnaik, Archita*
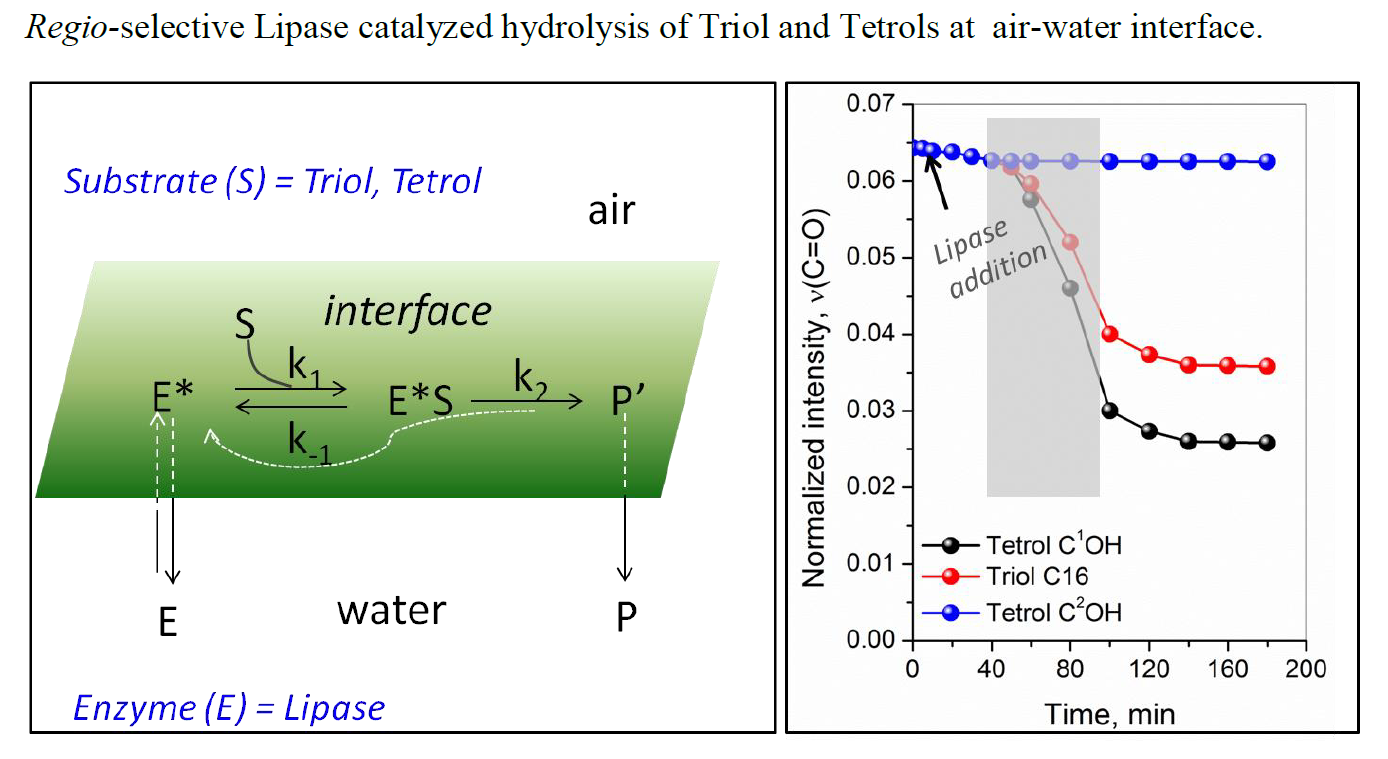
Interfacial hydrolysis of oxanorbornane-based amphiphile (Triol C16) by Candida rugosa lipase was investigated using real-time polarized infrared reflection absorption spectroscopy (IRRAS). The kinetics of hydrolysis process was studied by analyzing the ester carbonyl n(C=O) stretching vibration band across the two dimensional (2D) array of molecules in a confined environment. The enzyme-induced selective cleavage of the ester bond was spectroscopically monitored by the disappearance of the intense n(C=O) resonance at 1736 cm–1. Consequently, the in-situ spectroscopic measurements evidenced selective ester hydrolysis of Triol C16 yielding Tetrol C2OH and Palmitic acid, which remained predominantly in the un-dissociated form at the interface.
Chemistry and Physics of Lipids, 2017, 204, 25-33
Can Helical Peptides Unwind One Turn at a Time? – Controlled Conformational Transitions in a,b2,3 Hybrid Peptides
Dhayalan Balamurugan and Kannoth M. Muraleedharan*
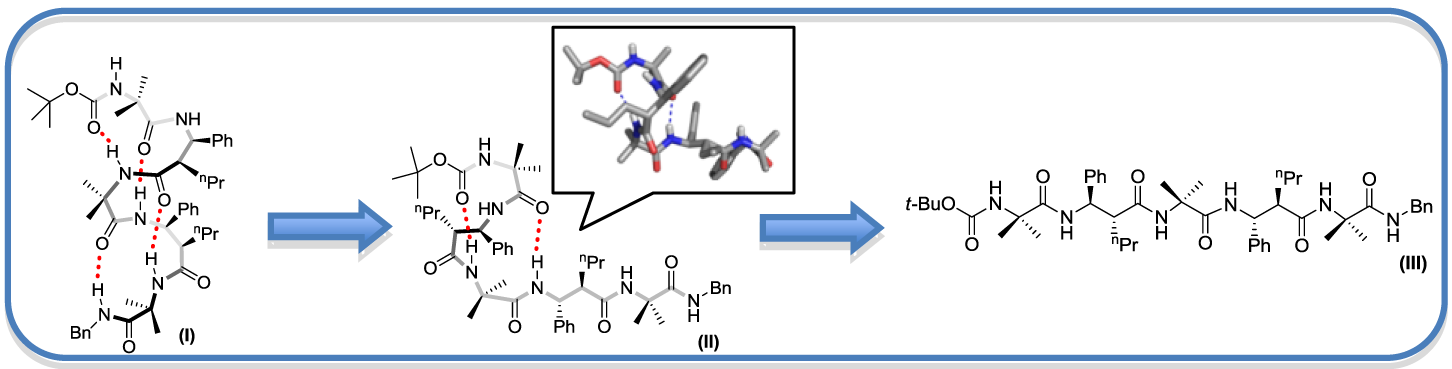
Unfolding of helical trans–b2,3-hybrid peptides with (a-b)na composition when executed by increasing solvent polarity or temperature, proceeded in a systematic manner with the turns unwinding sequentially; C-terminal region of these peptides were first to unwind and the process propagated towards N terminus with more and more b-residues equilibrating from gauche to anti- rotameric state across Ca-Cb. This is evidenced by clear change in their CH signal splitting, 3JCaH-CbH values, and sequential disappearance of i,i+2 NOEs.
Chem. Eur. J., 2015, 21 (26), 9332-9338
Conformational Switching in Heterochiral a,b2,3-peptides in Response to Solvent Polarity
Dhayalan Balamurugan and Kannoth M. Muraleedharan*
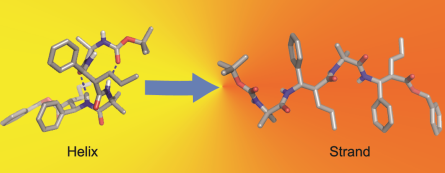
a,b-hybrid peptides with heterochiral backbone showed the ability to exist in helical-, extended- or partially folded conformations depending upon the solvation condition employed.
Eur. J. Org. Chem. 2015, 24, 5321-5325
Peptide turns through just ‘one atom’! A sulfamide group nucleates folding and stabilizes new supramolecular topologies in short peptides
Pushparathinam Gopinath, Venkatachalam Ramkumar and Kannoth Manheri Muraleedharan*
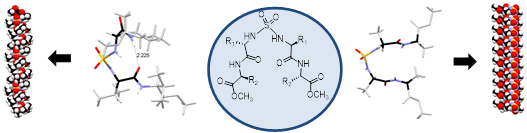
Peptide segments with centrally placed sulfamide groups showed remarkable tendency to adopt turn conformation and exhibited supramolecular topologies like ‘helical stacks’ and ‘hairpin sheets’ through highly co-ordinated array of strong and weak hydrogen bonds.
CrystEngComm, 2014, 16, 10371-10375
Chemical Environment as Control Element in the Evolution of Shapes – ‘Hexagons and Rods’ from an 11-Helical a,b2,3-hybrid Peptide
Dhayalan Balamurugan and Kannoth M. Muraleedharan*
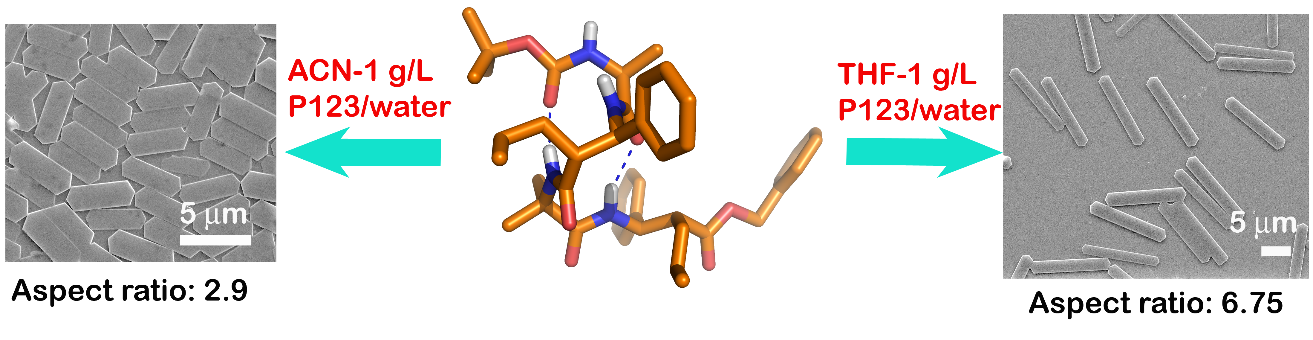
Chemical environment-dependant supramolecular assembly of 11-helical a,b2,3-hybrid peptide which give hexagonal or rod-like microcrystalline structure is demonstrated. Apart from the modulatory role by P123, sequential use of organic co-solvents during the initiation and propagation phases is shown to contribute to the size and shape control during molecular organization.
Soft Matter 2012, 8, 11857-11862
Unprecedented Torsional Preferences in trans–b2,3-Amino acid Residues and Formation of 11-Helices in a,b2,3-Hybrid Peptides
Dhayalan Balamurugan and Kannoth M.
Muraleedharan*
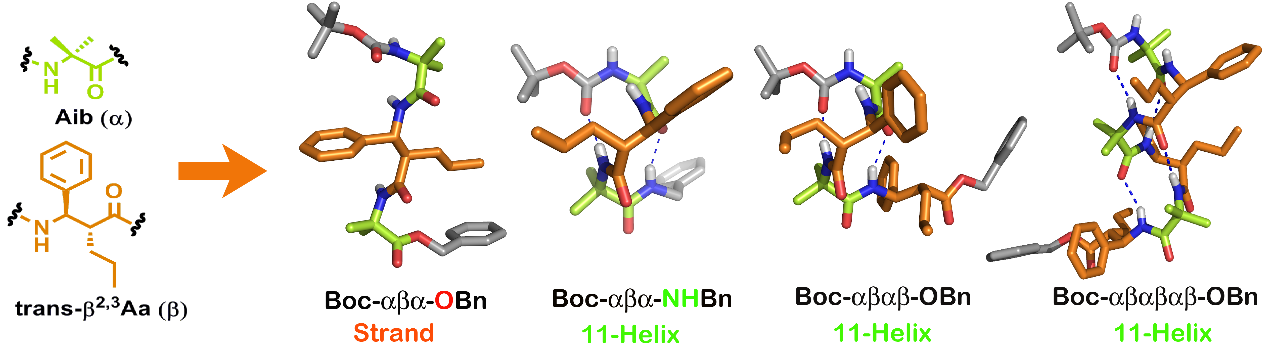
Anti or gauche? Trans-b2,3-amino acid residues which are known, thus far, to promote extended structures in their peptides showed specific rotamer preferences in response to intramolecular hydrogen bonding possibility to facilitate 11-helical structures in their 1:1 a,b-hybrid peptides. Gauche preference for all internal b-residues and anti- preference for the C-terminal residue in these peptides were confirmed through NMR and X-ray diffraction experiments.
Chem. Eur. J. 2012, 18, 9516-9520
Trans-b 2,3–amino acid-based Supramolecular Synthons for Probing the Interrelationships Between Structure, Torsion-directed Assembly and Isomorphism
Balamurugan D., Ramkumar V. and Muraleedharan K. M.*
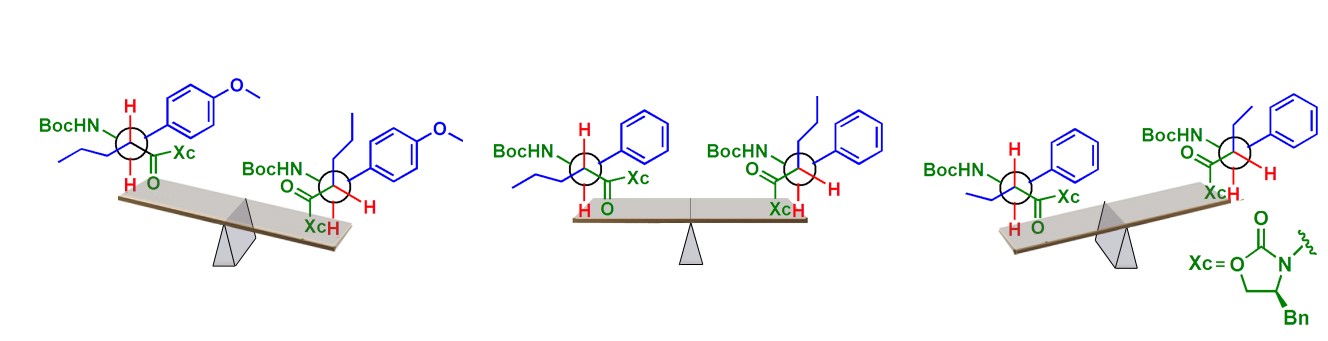
Variation in lattice arrangement and tendency towards isomorphic behavior in a group of trans-b2,3 amino acid derivatives with Boc- and oxazolidinone moieties at N- and C-terminals is discussed. Substitution pattern at a– and b positions in these systems was found to give different torsional preferences and hence different molecular organizations in their crystals. Analysis of such preferences in their azide analogs has unraveled the involvement of a relatively uncommon carbonyl-azide dipolar interaction in lattice stabilization.
Crystal Growth and Design, 2010, 10 (6), 2460–2464
Multi-chelation approach towards natural product-like skeletons: one-pot access to a nitrogen-containing tetracyclic framework from AlaAla dipeptide
Kasipandi, V.; John Victor N.; Ramkumar, V. and Muraleedharan K. M.*
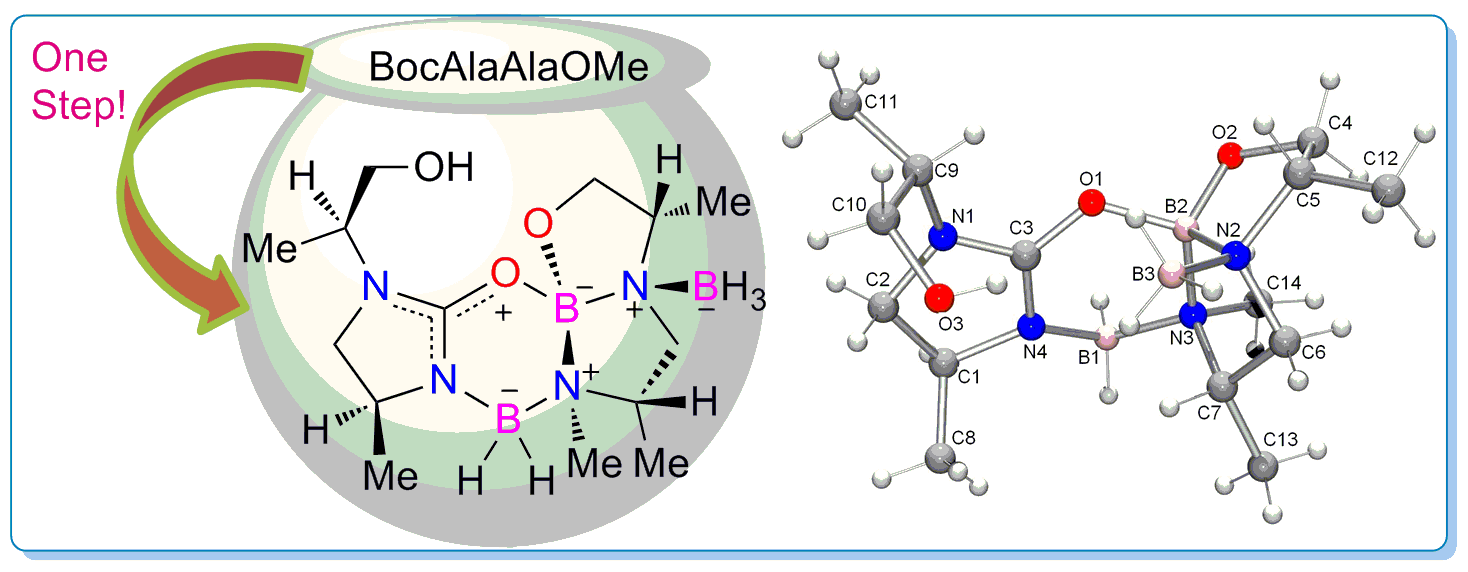
Reductive transformation of the dipeptide BocAlaAlaOMe to a complex, internally charge-stabilized, natural product-like skeleton in one synthetic step is discussed. Stepwise replacement of B-H bonds in borane by B-N or B-O incorporated three boron atoms in a tetracyclic framework of which one is stereogenic!
Chem. Commun., 2010, 46, 9212-9214
Gel-based Supramolecular ON-OFF Switch from Aryl-triazolyl Peptides with Excellent Chiro-optical-, Thixotropic-, and Self-healing Characteristics
Bhartendu K. Srivastava and Kannoth M. Muraleedharan *
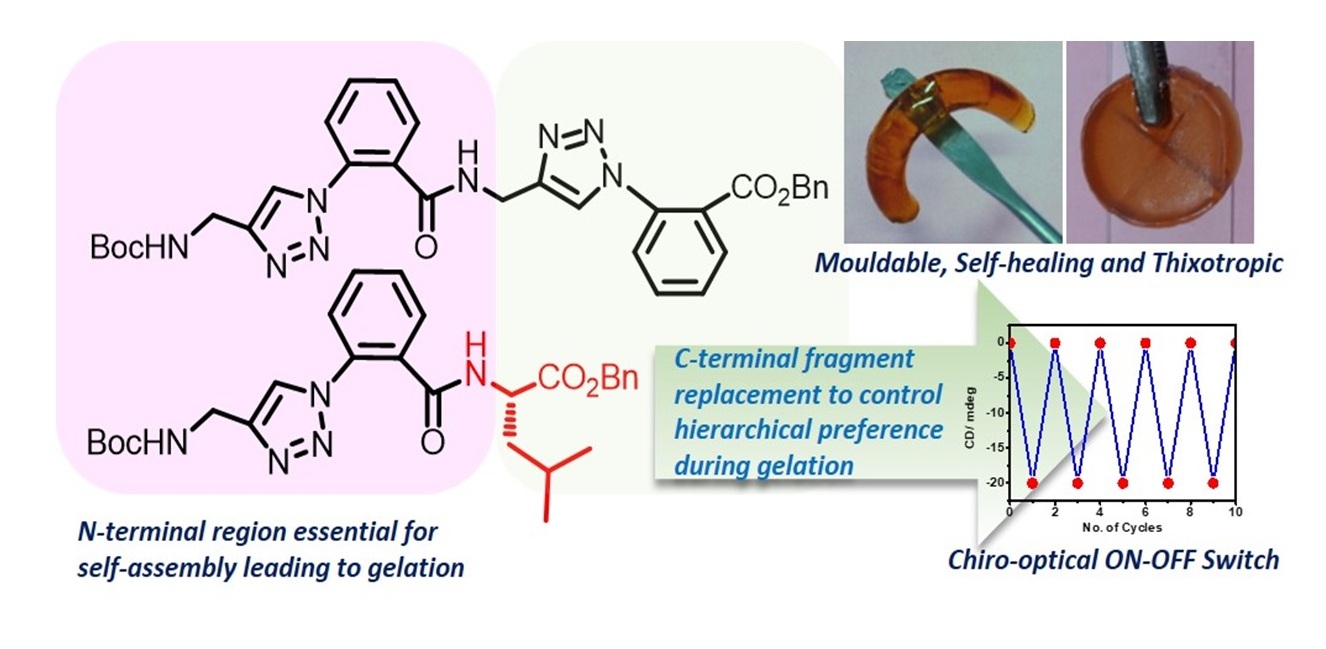
Systematic structure-property optimization of an achiral gelator (1.0) through a fragment replacement approach led to the identification of a new chiral system (1.4) which exhibit consistent and perfectly reversible chiro-optical response on Sol-Gel transition that can work like an ON-OFF switch. It was also able to direct the assembly of its achiral precursor 1.0 to give similar CD responses. In addition, its gels are mouldable, self-healing and highly thixotropic.
Soft Matter, 2018, 14, 1631-1636
Towards a fragment-based approach in gelator design: halogen effects leading to thixotropic, mouldable and self-healing systems in aryl-triazolyl amino acid-based gelators
Bhartendu K. Srivastava and Kannoth M. Muraleedharan *
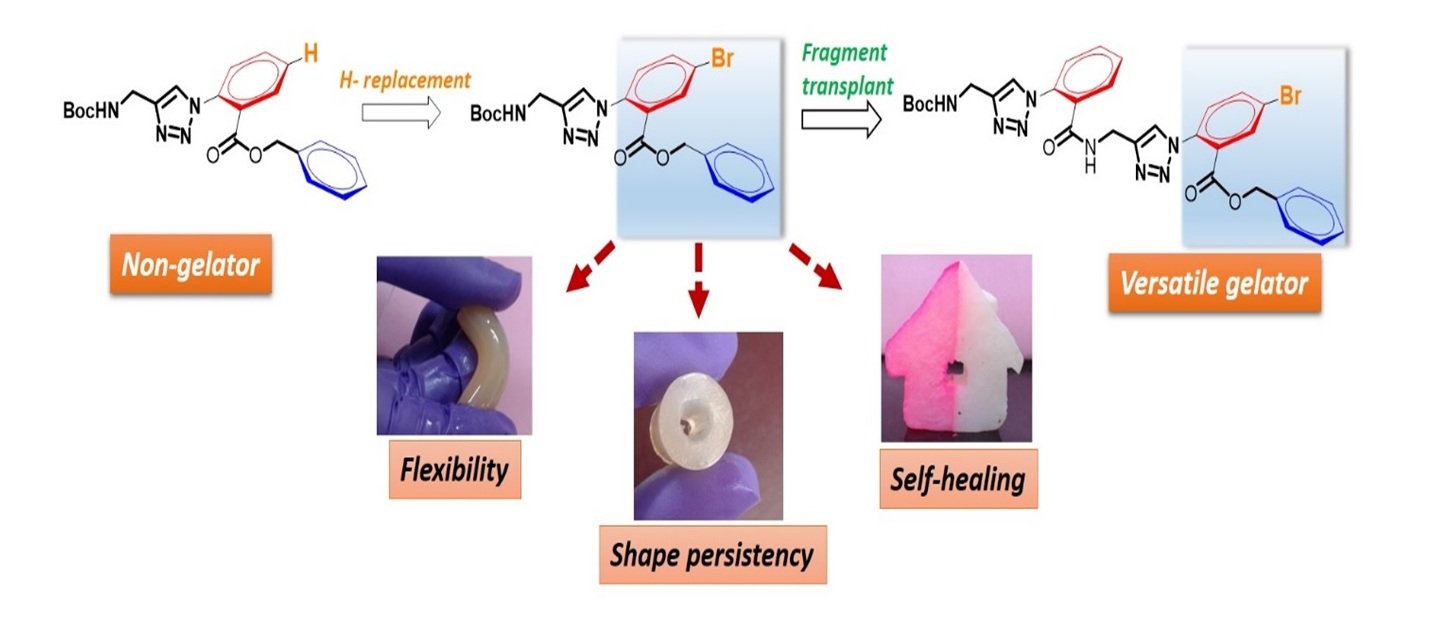
Simple replacement of a H atom by Br transformed the non-gelating Aryl triazolyl amino acid to a versatile gelator, which forms shape-persistent, self-healing and mouldable gels. The ‘bromo-aryl benzyl ester’ fragment was then transplanted to another framework, which resulted in similar solvent preference and gelation efficiency.
Chem. Commun. 2017, 53, 4485-4488
Sulfamide Lattice Restructuring to Dimensionally Controlled Molecular Arrays and Gel-forming Systems
S. V. Raghava, P. Gopinath, Bhartendu K. Srivastava, V. Ramkumar, and K. M. Muraleedharan *
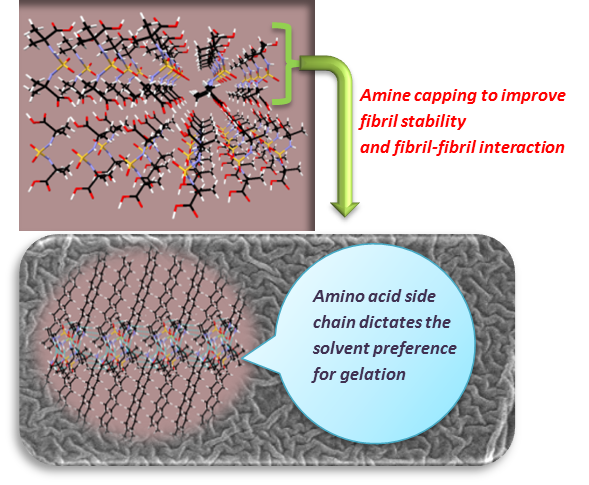
A design approach that incorporates structural requirements for 1D-assembly, fibril stability and fibril-fibril interaction for gelation was attempted using amino acid-based sulfamides with the general structure Aa-NH-SO2-NH-Aa. Preference for 1D-assembly alone was not a sufficient condition for gelation which became evident from studies involving sulfamide esters 1-5. Reducing the crystallization tendency without hindering unidirectional growth was executed through their diacids in combination with various amines capable of forming envelope around the sulfamide core through salt bridges. This strategy was fruitful, and gels of a wide variety of solvents could be made by varying the acid and amine components. Use of dodecylamine or benzylamine, which could stabilize the molecular layers through alkyl chain segregation or p-p interaction improved the gelation tendency, while the nature of amino acid side chain, especially its rotational freedom and hydrophobicity had a direct role in dictating the solvent preference. Crystallographic studies involving these two-component systems gave molecular level insights into the assembly, and showed the importance of anisotropy in the distribution of secondary interactions in gelation.
Chem. Eur. J. 2017, 23, 3658-3665
Aryl-triazolyl peptides for efficient phase selective gelation and easy removal of dyes from water
Bhartendu K Srivastava and Kannoth Manheri Muraleedharan *
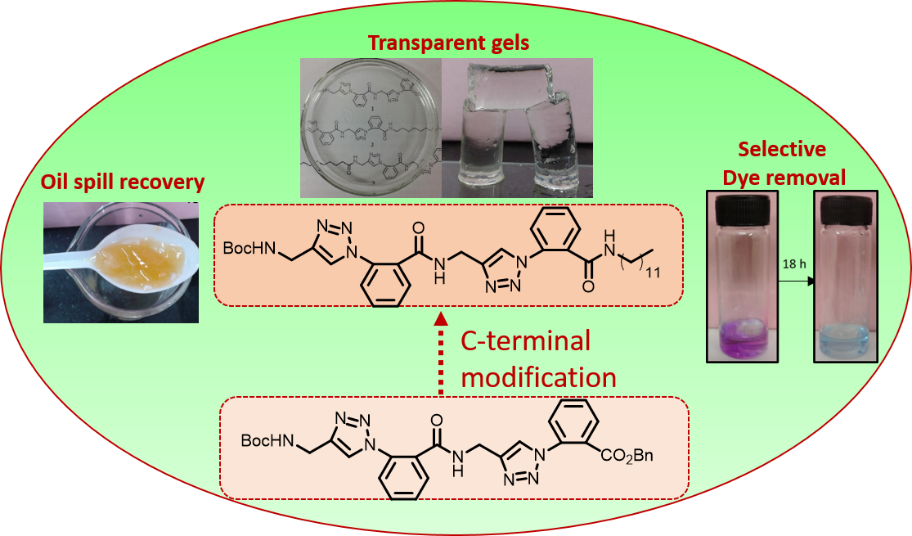
Fine-tuning the gelation ability of aryl triazolyl peptide 1 by C-terminal modification led to the identification of 2 with remarkable ability to form highly transparent gels in a wide range of solvents including oils. Good rheological properties, effective phase-selective gelation in short time periods, and usefulness in dye removal from water are the other highlights.
RSC Adv. 2016, 6, 29197-29201
A Modular Approach Towards Drug Delivery Vehicles Using Oxanorbornane-based Non-ionic Amphiphiles
D. Sirisha Janni, U. Chandrasekhar Reddy, Soumya Saroj , K. M. Muraleedharan *
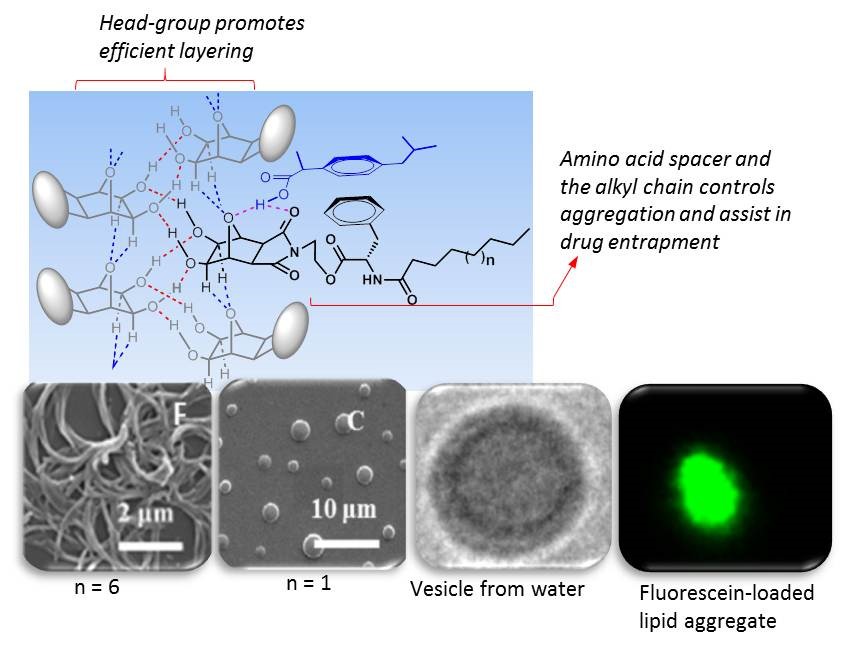 The self-assembly of non-ionic amphiphiles with hydroxylated oxanorbornane head-group was controlled using amino acid units as spacer between hydrophilic and lipophilic domains to get spherical supramolecular aggregates. Ability of these systems to harbour therapeutic agent like ibuprofen, and their drug-release profiles were evaluated. Apart from directing the assembly, the intervening amino acid unit was found to help in drug entrapment as well. Presence of cholesterol improved their drug-loading ability, and encapsulation efficiency of upto 66% was shown by the formulation containing the phenylalanine residue as the spacer (NC1c). There was no burst release, and 45% drug release was observed at the end of 24 h in this case (cf. soyaphosphatidylcholine based formulation = 49%).
The self-assembly of non-ionic amphiphiles with hydroxylated oxanorbornane head-group was controlled using amino acid units as spacer between hydrophilic and lipophilic domains to get spherical supramolecular aggregates. Ability of these systems to harbour therapeutic agent like ibuprofen, and their drug-release profiles were evaluated. Apart from directing the assembly, the intervening amino acid unit was found to help in drug entrapment as well. Presence of cholesterol improved their drug-loading ability, and encapsulation efficiency of upto 66% was shown by the formulation containing the phenylalanine residue as the spacer (NC1c). There was no burst release, and 45% drug release was observed at the end of 24 h in this case (cf. soyaphosphatidylcholine based formulation = 49%).
J. Mater. Chem. B, 2016, 4, 8025 – 8032
Tailoring strained oxanorbornane headgroups to dimensionally controlled nanostructures through hydrogen bonding
Nivarthi Ramesh, M. Ganesan, Nirod Kumar Sarangi, K. M. Muraleedharan and Archita Patnaik*
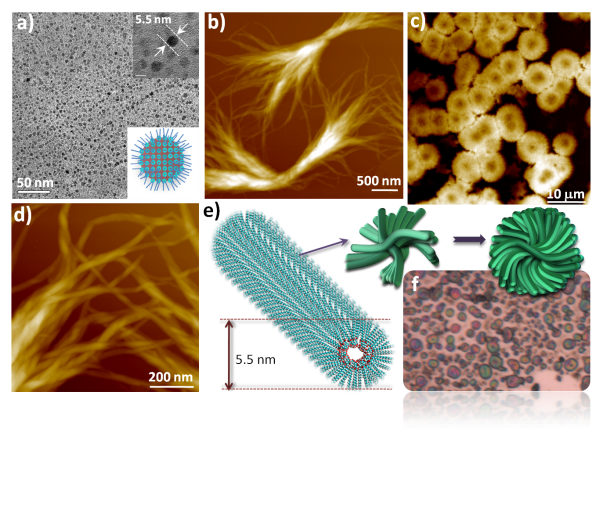
Amphiphilic oxanorbornane skeletal frame efficiently organized to direct the formation of dimensionally controllable, geometry-specific nanostructures as diverse as 0-D surface/reverse micelles, 1-D nanofibers, 2-D rectangular /square sheets, and 3-D flowers. The racemate with its precise stereo-projection of the -OH groups and the ring oxygen catered towards Li+ ion sensing and aided in the formation of pre-micellar aggregates of conventional surfactants.
RSC Adv., 2014, 4, 9762-9770
Hierarchical Preferences of Hydroxylated Oxanorbornane-Based Achiral Amphiphiles
D. Sirisha Janni and Muraleedharan K. Manheri *
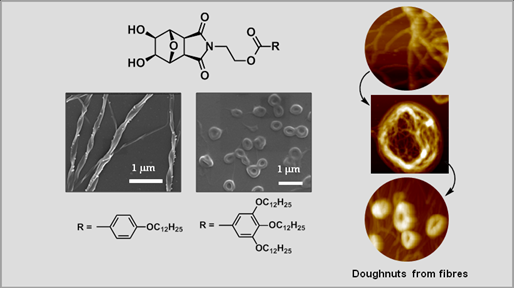
Achiral amphiphiles with hydroxylated oxanorbornane head groups showed specific morphological characteristics and hierarchical preferences depending upon the nature of lipophilic units. Detailed SEM studies showed that twisted ribbon-like aggregates are characteristic of mono-alkoxyaryl lipids with hydrocarbon chain length in the range of C10-C13; these systems also had a preference towards lamellar arrangement. Asymmetric packing of these lipids is a unique occurrence and shows that presence of molecular chirality is not an absolute requirement for curvature effects in their supramolecular assemblies. Aryl units in these systems were found important for the observed morphological preferences which became evident from comparative studies involving simple long chain esters without this moiety. Single crystal X-ray diffraction analysis of one of the lipids from the latter group gave finer details of strong and weak secondary interactions which operate during their assembly process. Introduction of more than one alkyl chain on the aromatic ring caused a notable shift in the packing propensity towards columnar arrangement. Most of these cone-shaped molecules were found to give doughnut-shaped aggregates from acetone solution through the intermediary of fibrous structures which was confirmed through SEM, TEM and AFM studies.
Langmuir, 2013, 29 (49), 15182–15190
Oxanorbornane-based Amphiphilic Systems: Design, Synthesis and Material Properties
M. Ganesan and K. M. Muraleedharan*
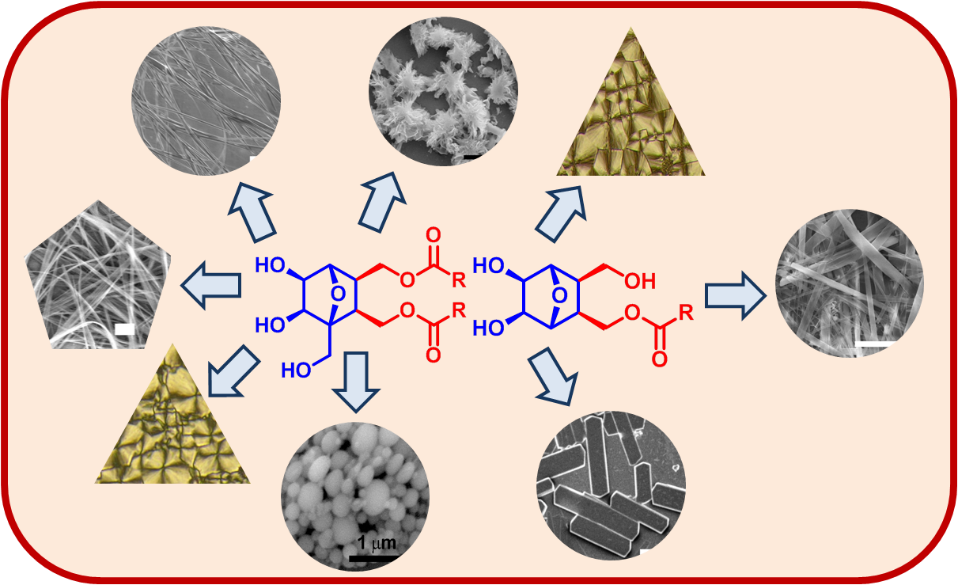
Use of oxanorbornane-based sugar-like surfaces in the development of new amphiphilic systems is demonstrated. Crystallographic analysis and results from aggregation studies showed their close resemblance to glycolipids.
RSC Advances, 2012, 2, 10, 4048-4051

